Genome Wide Association Studies: In recent years, genome wide association studies (GWAS) have become a mainstay in human genetics, plant and animal breeding, and disease research. But what exactly are they? How do they work? What are their strengths and weaknesses? And how do they tie into prediction methods and mapping?
In this blog we will explore what is genome wide association study, genome wide association mapping, genome wide association analysis, and how genome wide association studies and genomic prediction interplay. We will also discuss the benefits and limitations of genome wide association studies, and wrap with examples, quotes, and interactive questions to keep things alive.
Imagine you are a researcher wanting to find genetic variants that influence height, diabetes risk, or yield in crops. You have genotype data (millions of SNPs) and phenotype data (trait measurements) for many individuals. How do you connect contributions of genetic variants scattered across the genome to traits? That is precisely the goal of genome wide association studies.
In simple terms, GWAS provide a hypothesis-free framework: you scan across the genome (not limiting to candidate genes) to detect associations between genetic markers and traits. Because of high throughput SNP arrays, sequencing, and big biobank data, genome wide association studies have become feasible and powerful.
Read More: Drug Safety Associate Jobs | Role, Skills, Interview, Career in India
One large review state:
“Genome-wide association studies compare common genetic variants in large numbers of affected cases to those in unaffected controls to determine whether an association with disease exists.”
Thus, genome wide association studies are now foundational in modern complex-trait genetics.

What are Genome Wide Association Studies?
A genome wide association study is an observational study in which researchers scan a genome-wide set of genetic markers (often millions of SNPs) in many individuals in order to find variants (alleles) that associate statistically with a trait or disease.
Important points:
- It is not limited to preselected candidate genes; it is agnostic across the genome.
- It uses common variants (such as SNPs) as markers.
- The design often is case vs control for diseases, or uses continuous trait measurements.
- After scanning, one finds SNPs with allele frequency differences (or effect sizes) correlated with trait values.
As the US National Human Genome Research Institute describes:
“Genome-wide association studies involve scanning markers across the genomes of many people to find genetic variations associated with a particular disease.”
Hence the name: genome wide (across entire genome) and association studies (statistical correlation between genotype and phenotype).
Steps in Genome Wide Association Mapping / Analysis
When you read about genome wide association mapping or genome wide association analysis, these refer to the process and techniques used in GWAS. Let us break down the pipeline:
Study design & sample collection
- Decide on cases and controls, or cohorts measured for a quantitative trait.
- Ensure suitable sample size for power (often thousands to tens or hundreds of thousands).
- Collect phenotype data carefully (e.g. disease status, traits).
- Genotype the individuals at many SNPs.
Quality control (QC)
- Filter out poor-quality SNPs (low call rate, Hardy–Weinberg violations).
- Filter individuals (missing data, relatedness, population stratification).
- Impute missing genotypes to increase SNP coverage (imputation using reference panels).
- Control for population stratification (ancestry differences) via PCA or mixed models.
Association testing
- For each SNP, test association with trait (e.g. logistic regression for case/control, linear regression for quantitative trait).
- Include covariates (age, sex, principal components) to adjust confounding.
- Get p-values, effect sizes (beta or odds ratio), standard errors.
Multiple testing correction & significance threshold
- Because millions of tests are done, we must correct for multiple comparisons (e.g. Bonferroni, FDR).
- Standard threshold often used is
in human GWAS (to maintain genome-wide significance).
Visualization & interpretation
- Use Manhattan plots (–log10 p-value vs genomic position) to highlight significant SNPs.
- Use QQ plots to see inflation or p-value distribution.
- Map SNPs to genes or regulatory regions (annotation).
- Use fine mapping, functional annotation, or follow-up experiments to narrow causal variants.
Replication & validation
- Significant SNPs are tested in independent cohorts to see if associations replicate.
- If replicated, the findings gain credibility. (Replication is essential in genome wide association studies)
Post-GWAS analyses
- Polygenic risk score construction.
- Genetic correlation across traits.
- Mendelian randomization.
- Functional follow-up (gene expression, regulatory assays).
- Pathway enrichment, network analysis.
This entire pipeline is usually referred to as genome wide association analysis or genome wide association mapping (mapping the trait to variants).
In short, genome wide association studies are not just the test step, they entail careful design, QC, testing, and downstream interpretation.
Statistical foundations
To see why and how genome wide association studies work, we examine statistical foundations.
Linear / logistic regression per SNP
At core, for each SNP, we test:
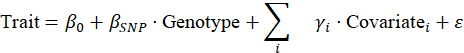
- In case/control: logistic regression yields odds ratio.
- In quantitative traits: linear regression yields beta (effect per allele).
The null hypothesis is . P-value tells significance of association.
Multiple testing & significance threshold
Because of large number of SNPs, many false positives may arise. Thus, a stringent threshold (e.g. ) or FDR control is adopted. This is key in genome wide association studies to avoid spurious hits.
Power and effect sizes
Many causal variants have small effect sizes; large sample sizes are needed to detect them. Some effects are too weak to be picked by GWAS unless sample sizes are very large.
Missing heritability & polygenicity
GWAS results often explain only a small fraction of heritability estimated from family/twin studies. Many variants may have too small effect sizes or be rare, or structural variants may be missed. This gap is known as missing heritability.
Traits are often polygenic: many variants each contributing small amount.
Mixed models and control of stratification
To manage confounding via ancestral differences, linear mixed models (LMMs) or methods like MLM (mixed linear model) are used. These incorporate kinship matrices to reduce false associations due to population structure.
Fine mapping, colocalization, Bayesian methods
After initial association, Bayesian methods or model selection approaches can help identify likely causal variants among correlated SNPs. (E.g. Bayesian variable selection regression in GWAS)
Thus the statistical basis of genome wide association studies is robust but demands careful design and corrections.
Genome Wide Association Studies and Genomic Prediction
An important application of genome wide association studies is combining them with prediction frameworks; this is where genome wide association studies and genomic prediction converge.
Genomic prediction: what is it?
Genomic prediction is the use of genotype data (many markers) to predict trait values (phenotypes) in new individuals. This is widely used in animal and plant breeding. It often uses methods like GBLUP, ridge regression, Bayesian models, or machine learning models.
How GWAS informs prediction?
GWAS yields effect estimates for SNPs. These can serve as inputs or weights in predictive models. Some models use SNPs that pass significance; others use all SNPs with shrinkage (even those not reaching genome-wide cutoff). The idea: combine association signals (strong or weak) to build prediction models.
Advantages & pitfalls
- GWAS helps identify informative SNPs that can be prioritized.
- However, prediction models often perform better when using all SNPs with shrinkage rather than only genome-wide significant ones, because many weak variants still carry predictive power.
In plant and animal breeding, combining genome wide association studies and genomic prediction is common: first do GWAS, discover candidate SNPs/regions, then build prediction models for breeders.
Thus, genome wide association studies play a dual role: (i) discovery of trait loci, and (ii) feature selection in predictive models.
Benefits and Limitations of Genome Wide Association Studies
No method is perfect. Let us ask: What are the benefits and limitations of genome wide association studies? Here are key points:
Benefits (advantages)
- Unbiased scanning across the genome, no need to preselect candidate genes.
- Ability to discover new, unexpected loci associated with traits/diseases.
- High resolution mapping (with dense SNPs, fine mapping, imputation).
- Works across many organisms (human, plants, animals).
- Once association signals are found, they yield biological hypotheses and functional follow-up opportunities.
- Enables polygenic risk scores, cross-trait genetic correlations, Mendelian randomization, etc.
Many authors commend that GWAS has “facilitated an impressive range of discoveries impacting multiple fields.”
Limitations (weaknesses / challenges)
- Many associations have small effect sizes, making them hard to detect.
- Missing heritability: GWAS explain only a fraction of total genetic variance estimated from family studies.
- Rare variants, structural variation, copy number changes may be missed because GWAS typically uses common SNPs.
- Population stratification can produce false positives if not properly controlled.
- Because of multiple testing burden, the significance thresholds are very stringent; many true associations are lost.
- Correlation, not causation: GWAS identifies associations, not necessarily causal variants.
- The associated SNP might be a tag in linkage disequilibrium; the real causal variant may be elsewhere.
- Generalisability across populations: Many GWAS are done in European populations; signals may not transfer to other ancestries.
- Requires large sample sizes and high cost in genotyping/imputation.
- Interpretation and functional validation are challenging: connecting variant to gene and mechanism is laborious.
As a review article puts it, the benefits and limitations of genome wide association studies must be weighed in any realistic plan.

Examples & Case Studies
To make things concrete, let us see how genome wide association studies have been used in practice.
Human disease traits
- GWAS for height, type 2 diabetes, heart disease, schizophrenia, Alzheimer’s disease, many SNP loci have been identified. (Over 3,000 human GWAS as of sometime)
- Example: the first GWAS in 2005 on age-related macular degeneration found two SNPs on complement factor H gene region.
- Many disease loci have been followed by functional experiments and therapeutic hypotheses.
Plant and crop breeding
- GWAS in rice, maize, wheat to find yield-related loci, disease resistance genes. (In plants, sometimes called GWA mapping)
- For example, genome wide association mapping in rice has revealed loci for grain size, plant height etc.
Integration with prediction
- In breeding, once GWAS loci are known, breeders build prediction models using SNPs from GWAS plus genome-wide SNP data to estimate breeding values. This is exactly a case of genome wide association studies and genomic prediction in action.
Deep learning & GWAS
- Recent works use deep learning models on GWAS SNP sets to classify traits (for example, obesity prediction using SNPs from GWAS)
- This shows the fusion of genome wide association analysis with modern machine learning methods.
These examples show how genome wide association studies lead to real discoveries and actionable insights.
What Lies Ahead for Genome Wide Association Studies?
- Inclusion of diverse ancestries in GWAS to improve generalisability and reduce bias. Many GWAS currently overrepresent European populations.
- Better modeling of rare variants, structural variants, copy number variants, epigenetic features.
- Integrating multi-omics data (transcriptomics, epigenomics, proteomics) to move from association to mechanism.
- Use of meta-analyses combining multiple cohorts to improve power.
- Improved fine mapping and causal inference (colocalization, Mendelian randomization).
- Methods to deal with gene–gene interaction (epistasis) and gene–environment interaction.
- Combining genome wide association mapping with genome wide association studies and genomic prediction more tightly, especially in breeding contexts.
- Ethical, legal, and social implications: data privacy, consent, population stratification across human groups, return of results.
- Better interpretability: moving from associated SNP to functional genes, regulatory elements, and biological mechanism.
- Machine learning/AI integration: using GWAS results as input to models for classification or phenotype prediction.
Challenges remain, especially regarding benefits and limitations of genome wide association studies: small effect sizes, missing heritability, translation into biology, and generalisation to diverse populations.
On A Final Note…
Let me summarise the whole thing for you here:
- Genome wide association studies are observational studies scanning the whole genome’s genetic markers to find statistical associations with traits.
- When someone asks what is genome wide association study, the succinct answer is: a genome-wide scan for genotype–phenotype correlations.
- Genome wide association mapping or genome wide association analysis refer to mapping and analyzing those associations via regression models, correction, QC, interpretation.
- We also looked at how genome wide association studies and genomic prediction combine discovery and predictive modeling.
- The benefits and limitations of genome wide association studies include unbiased locus discovery but issues like missing heritability, small effect sizes, and interpretation challenges.
- Examples in human disease and plant breeding show how GWAS works in practice.
- The future holds promise in integrating multi-omics, diverse populations, rare variants, and stronger causal inference.

