All You Need To Know About Regulatory Affairs Career Path: Regulatory affairs is an emerging career path for someone from the life sciences and health-related backgrounds. As the bridge between the pharmaceutical industry and regulatory authorities, professionals in this field ensure that products like drugs, medical devices, and biotechnology products comply with regulations.
And with the rise of the clinical research industry in India, the regulatory affairs career path has become increasingly sought after – and with the right reasons – which is what we will get to in this blog by LLRI.
This blog will cover the following:
- Regulatory affairs career path
- Regulatory affairs career options
- Regulatory affairs career development
- Regulatory affairs career growth
Regulatory Affairs Career Path: Overview
- Regulatory affairs career path offers several job opportunities and career growth potential.
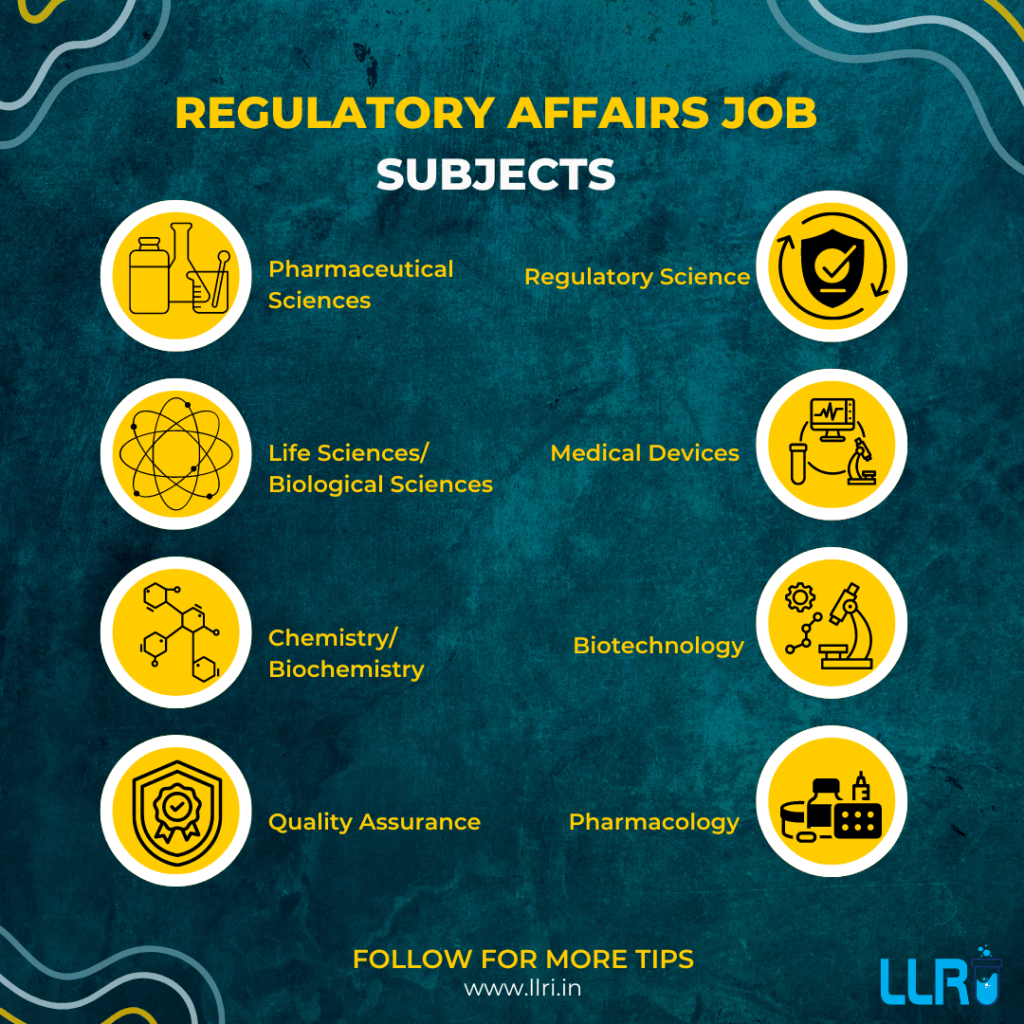
- For a regulatory affairs job, starting with a strong educational background and specialized training, such as a clinical research course, can set the foundation for a successful career.
- Continuous professional development and advanced certifications are important for career advancement.
- The demand for regulatory affairs professionals is increasing, providing ample opportunities for career growth.
What Is Regulatory Affairs?
Before getting into the details, regulatory affairs is a field that ensures companies comply with all of the regulations and laws pertaining to their business. For the pharmaceutical, biotech, and medical device industries, this means ensuring that products are safe, effective, and in line with regulatory standards before they reach the market. The role of regulatory affairs professionals is critical to the success of any product, making it a highly sought-after career path.
Why Choose a Career in Regulatory Affairs?
Choosing a regulatory affairs career path offers several benefits. This profession is not only stable but also provides the opportunity to work on a wide range of projects that can have a significant impact on public health.
Additionally, as the global healthcare landscape continues to expand, the need for regulatory affairs professionals will only increase, offering job security and a promising future.
Regulatory Affairs Career Path: What You Should Know
A regulatory affairs career path is not just limited to instilling compliance with regulations but also involves interacting with government agencies, monitoring the progress of clinical trials, and making sure that safety and efficacy data are robust.
The roles can vary widely depending on the industry, such as pharmaceuticals, medical devices, or biotechnology. Typically, regulatory affairs professionals are responsible for submitting regulatory documents, developing regulatory strategies, and assuring that all products meet the local and global regulatory standards.
A career in regulatory affairs typically begins with getting a relevant degree, such as a life sciences or pharmacy degree, and often includes gaining more specialization through certifications or courses offered by a Clinical research institute or Clinical research training center.
If you are interested in specializing further, pursuing a clinical research course or enrolling in a PG Diploma in Clinical Research from the best institute for PG Diploma in Clinical Research can provide a strong foundation in this field.
What About Regulatory Affairs Career Options?
There are numerous regulatory affairs career options available in India and globally. Some of the key roles include:
- Regulatory affairs specialist: Focuses on preparing and submitting regulatory filings, such as New Drug Applications (NDAs) and Investigational New Drug Applications (INDs).
- Regulatory affairs manager: Manages the regulatory team, develops strategies for product approval, and maintains ongoing compliance.
- Regulatory affairs associate: Supports regulatory specialists and managers by helping prepare submission documents and making sure all documentation is accurate.
- Global regulatory affairs associate: Manages the submission and approval of products in multiple countries, ensuring that the products comply with the regulations in each market.
- Quality assurance and regulatory compliance officer: Makes sure that both products and processes meet the required regulatory standards and that all manufacturing and distribution processes are in line with regulatory requirements.

The other jobs you can get in terms of regulatory affairs is as follows:
- Regulatory affairs director
- Compliance specialist
- Food safety inspector
- Clinical research associate
- Director of quality assurance
These job roles can be found across various industries, including pharmaceuticals, biotech companies, clinical research organizations (CROs), and medical device companies. Moreover, each of these roles offers unique challenges and opportunities, making regulatory affairs career development an exciting journey.
Is Regulatory Affairs Career Growth Possible?
One of the most common questions people have been whether regulatory affairs career growth is possible. The answer is a loud yes. Regulatory affairs is a dynamic field with several opportunities for advancement.
As you gain experience and expand your knowledge, you can move into higher positions with more responsibility and higher pay.
Investing in continuous education is key to regulatory affairs career development. Enrolling in a clinical research course or attending a clinical research institute can provide you with the necessary skills and knowledge to advance your career. Additionally, getting certifications specific to regulatory affairs can significantly improve your prospects.
Regulatory Affairs Career Development
It is no surprise that regulatory affairs career development is important for those looking to progress in this field. Continuous learning and professional growth can be achieved through advanced certifications and specialized training. A clinical research training program, for instance, can offer in-depth knowledge and skills necessary for career advancement.
Networking with professionals in the field and staying updated with industry trends also play a significant role in career development. Consider joining professional organizations and attending industry conferences to enhance your job prospects as the additional certifications can always work in favour to you landing your desired job.

So, What Have We Learnt?
If you’re considering a career in regulatory affairs, there’s a clear and rewarding regulatory affairs career path ahead of you. Focusing on regulatory affairs career options and investing in regulatory affairs career development can position yourself for long-term success and growth in regulatory affairs field.
For those interested in improving their expertise, exploring options at a reputable clinical research institute or enrolling in clinical research training programs can be a strategic move. Embrace the opportunities and prepare for a thriving career in regulatory affairs!
Connect with us today to learn more!