What Is SDTM Programming? Data management is the root of accuracy and reliability. When it comes to clinical trials and healthcare, one of the primary aspects is SDTM programming. But what is it?
Let’s break it down step-by-step and learn about the important facts you must know about SDTM datasets and SDTM programming training.
This blog contains the following:
- What is SDTM?
- What is SDTM programming?
- What are SDTM datasets?
- Where is SDTM used?
- What is the importance of SDTM in healthcare?
- What jobs can I get if I’m skilled in SDTM?
What Is SDTM (Study Data Tabulation Model)?
The Study Data Tabulation Model, also known as SDTM, is a framework used in clinical trials to organize and present data in a standard format. SDTM was developed by the Clinical Data Interchange Standards Consortium (CDISC) to ensure that clinical trial data is uniformly structured and easily interpretable.
The SDTM standard helps regulatory bodies like the FDA and other healthcare organizations to review and analyze clinical trial data. In simple terms, SDTM provides a common language for clinical trial data, assuring that consistency and compliance across the industry is maintained.
What Is SDTM Programming?
Now, what is SDTM programming? It refers to the process of converting raw clinical trial data into SDTM datasets that adhere to the CDISC standards. SDTM programming involves a detailed understanding of both the clinical data and the specific SDTM guidelines.
These programming skills can help SDTM programmers create datasets that are ready for submission to regulatory authorities, which in turn makes sure that the data is clean, organized, and compliant with industry standards.
SDTM plays a crucial role in the success of clinical trials, as the accuracy of the datasets directly impacts the approval process of new drugs and treatments.
What Are SDTM Datasets? 5 Must-Know Facts
Fact 1: SDTM datasets are organized collections of clinical trial data that have been structured according to SDTM standards.
Fact 2: These datasets cover various aspects of a clinical trial, such as demographics, medical history, lab results, and adverse events.
Fact 3: Each SDTM dataset follows ad format and naming convention, making it easier to analyze and interpret the data.
Below is an example of a typical SDTM dataset format:
| Dataset Name | Description | Purpose |
| DM | Demographics | Contains patient demographics |
| AE | Adverse Events | Logs all adverse events during the trial |
| LB | Lab Results | Records laboratory test results |
| MH | Medical History | Tracks patients’ medical history |
Fact 4: SDTM datasets are basically the foundation for regulatory submissions.
Fact 5: It aids in the process of data collection, ensuring that the data collected during a clinical trial is standardized, accurate, and ready for analysis.
Where Is SDTM Used?
SDTM is primarily used in clinical trials, especially during the submission of data to regulatory authorities like the FDA or European Medicines Agency (EMA). It is also used by pharmaceutical companies, clinical research organizations (CROs), and clinical research institutes for managing and analyzing clinical data.
If you’re enrolled in a clinical research course or looking for a career in clinical data management, learning SDTM programming is very important. Clinical research training centres offer training to help you master the skills needed for the healthcare field.

What Is The Importance Of SDTM In Healthcare?
SDTM is central to healthcare because it sets up the clinical trail data in a consistent, standardized format. And it is this standardization that facilitates the proper analysis of trial results, making it easier for regulators to evaluate the safety and effectiveness of new treatments.
Moreover, SDTM programming is responsible for making that the data is traceable, auditable, and easily integrated into larger datasets for meta-analysis. And it also integrates the data submission process but also plays a critical role in bringing new therapies to market faster and more efficiently.
When it comes to the healthcare field as a whole, the importance of SDTM in healthcare cannot be overstated. Whether you’re pursuing a clinical research course or working at a clinical research training center, knowledge of SDTM is invaluable.
What Jobs Can I Get If I’m Skilled In SDTM?
Mastering SDTM can open up several career opportunities in the clinical research field for you. Here are some of the roles you can pursue if you are skilled in SDTM:
- SDTM programmer: As an SDTM programmer, you will be responsible for converting raw clinical data into SDTM datasets, ensuring that they meet regulatory standards.
- Clinical data manager: This role involves overseeing the collection, processing, and storage of clinical trial data. A strong understanding of SDTM is essential for this position.
- Regulatory affairs specialist: This role focuses on ensuring that all data submitted to regulatory agencies is compliant with standards like SDTM.
- Clinical research associate (CRA): CRAs monitor clinical trials to ensure that data is collected accurately and in accordance with SDTM guidelines.
- Biostatistician: Biostatisticians analyze clinical trial data, often working with SDTM datasets to draw meaningful conclusions from the data.
If you’re interested in any of these roles, consider enrolling in SDTM programming training at a clinical research training center. With the right skills and training, you can build a rewarding career in this dynamic field.
On A Final Note…
To become proficient in SDTM programming, enrolling in SDTM programming training is essential. Such training equips you with the technical skills needed to work with SDTM datasets, and it provides hands-on experience with real clinical trial data.
SDTM programming training is typically offered by specialized institutes like the best institute for PG Diploma in Clinical Research, where you can learn everything from the basics of SDTM to advanced programming techniques. These courses are often part of a broader clinical research course that includes SDTM training modules, and you can expect clinical research course fees to vary depending on the training provider.
FAQ On SDTM Programming
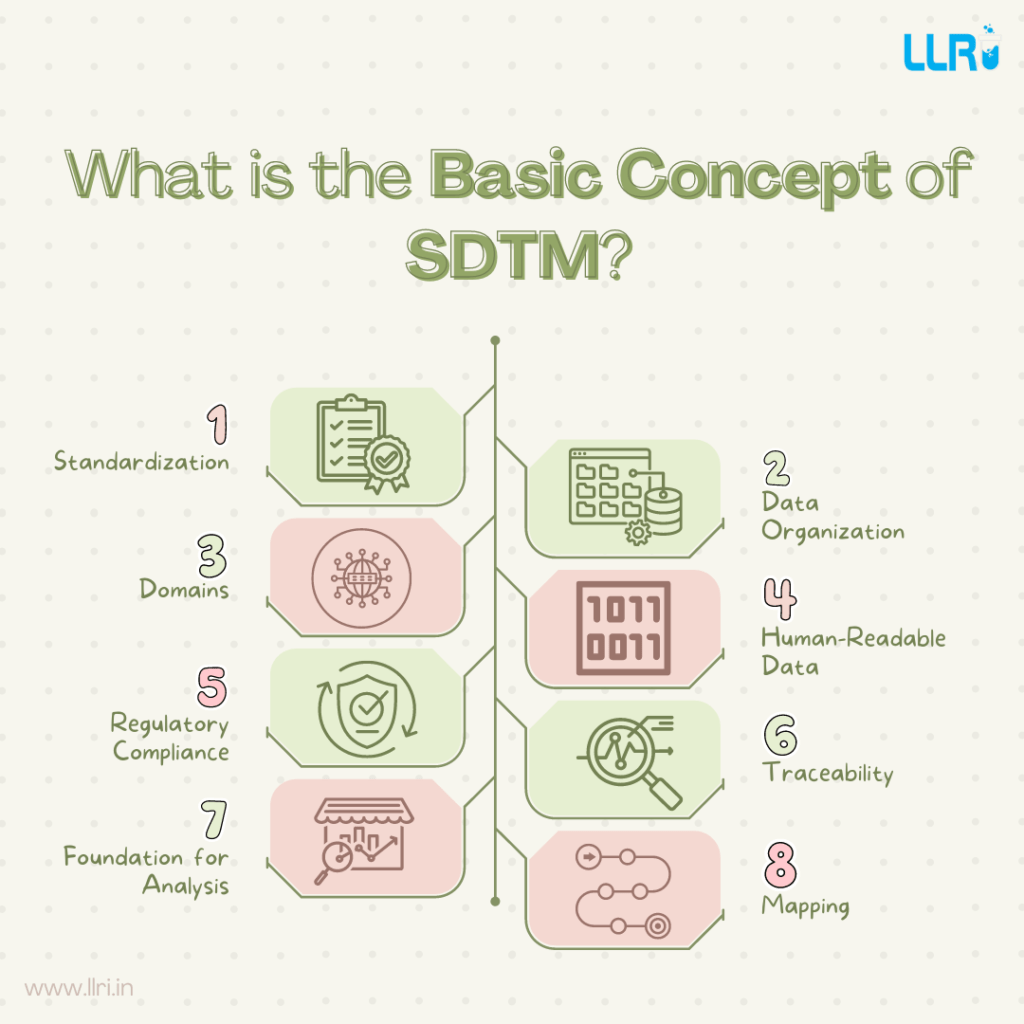
What is the basic concept of SDTM?
SDTM stands for Study Data Tabulation Model. It standardizes the format of clinical trial data to ensure consistency and compliance with regulatory standards.
What is the role of an SDTM programmer?
An SDTM programmer converts raw clinical trial data into SDTM datasets, ensuring that the data is organized, compliant, and ready for regulatory submission.
What does the SDTM stand for?
SDTM stands for Study Data Tabulation Model.
How to create SDTM datasets?
To create SDTM datasets, programmers use specialized software to map raw clinical data to SDTM-compliant formats, following the CDISC guidelines.