How To Become A Regulatory Affairs Professional: The regulatory affairs industry is centred around controlling the safety and effectiveness of healthcare products, that is, pharmaceuticals, medical devices, veterinary medicines, cosmetics, pesticides etc. Regulatory affairs focus on the discovery, testing, manufacture, and marketing of products aimed at ensuring safety and making a valuable contribution to public health.
These products are then certified by specially trained professionals to meet human standards, where the step-by-step process of development, testing, manufacturing, marketing, and distribution basically seals the safety and quality of the product.
A regulatory affairs professional is essential to global well-being, overseeing the development and distribution of safe, healthy food and medical advancements.
While they work across diverse fields like public policy, health, science, economics, and law, these professionals share a common mission: ensuring that safe and effective products reach the public for consumption and use.
Most regulatory affairs professional are employed in industries like pharmaceuticals, biotechnology, and food science, while others work as consultants in marketing, research, or legal firms.
Regulatory affairs professional can also be found in hospitals, healthcare institutions, clinical research organizations, academic settings, and government agencies. The scope of a regulatory affairs specialist’s responsibilities varies widely depending on the specific industry or organization in which they work.
Now that you have an understanding on the role of a Regulatory Affairs Professional, let’s get to the next step – How To Become A Regulatory Affairs Professional? What about regulatory affairs career change? Is regulatory affairs career progression possible? How to gain regulatory affairs professional development?
Let’s explore.

How To Become A Regulatory Affairs Professional? 8 Tips To Know
If you are planning to start a regulatory affairs career, like any other job, there are several important steps you should consider:
- Get a relevant degree
- Gain industry knowledge
- Obtain certifications
- Acquire work experience
- Try specialised training
- Develop key skills
- Stay updated with evolving industry knowledge
- And finally, start your career
- Get a relevant degree: Start with a bachelor’s degree in life sciences, pharmacy, chemistry, biomedical engineering, or a related field. A background in these areas will give you a solid foundation in science, research, and healthcare. You should also focus on getting an advanced degree as well.
- Gain industry knowledge: Learn about key regulatory bodies, such as the U.S. FDA, European Medicines Agency (EMA), and local regulatory authorities (these will be specific to each country). Also familiarise with the important guidelines Good Manufacturing Practices (GMP), Clinical Trial Regulations, and Medical Device Regulations.
- Obtain certifications: Pursue certifications such as the Regulatory Affairs Certification (RAC) offered by the Regulatory Affairs Professionals Society (RAPS). These certifications are globally recognized therefore can help add credibility to your profile. Enrolling in a clinical research course can help you out here, also help with regulatory affairs career change.
- Acquire work experience: To get the right level of work experience for regulatory affairs career progression, the first step is starting in roles like quality assurance, clinical trials, pharmacovigilance, or research and development to build a foundation in the industry. The next step would be to look for internships with companies or regulatory bodies to gain hands-on experience.
- Try specialised training: Consider earning a Regulatory Affairs Certification from the Regulatory Affairs Professionals Society (RAPS). Take short courses too, such as drug approval processes, medical device regulations, and labeling requirements.
- Develop key skills: This one goes without saying. You’ll need strong analytical skills to interpret complex regulations and ensure compliance for regulatory affairs professional development, along with good written and verbal communication skills. Basically, the key skills for a regulatory affairs professional are (a) regulatory knowledge (b) attention to detail (c) communication skills and (d) project management.
- Stay updated with evolving industry knowledge: Every industry is developing in the span of heartbeat, so it is extremely important that you stay updated if you are looking for regulatory affairs jobs. And with the regulatory requirements constantly changing, stay updated by continuously learning through courses, certifications, and keeping up with industry news.
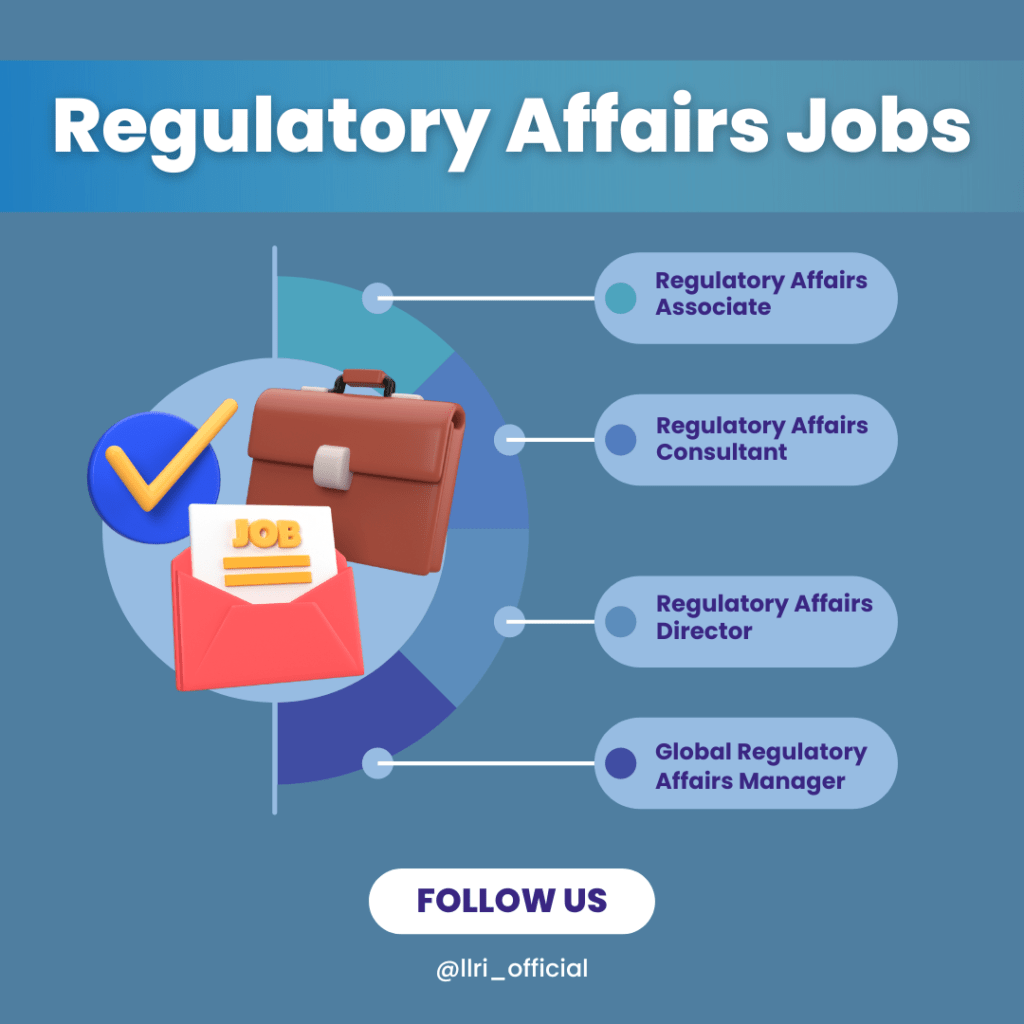
- Last but not least, start your career: Look for positions such as Regulatory Affairs Assistant or Specialist. These roles involve supporting regulatory submissions, maintaining regulatory documentation, and ensuring compliance with applicable regulations. With experience, you can move into roles like Regulatory Affairs Manager, Director, or Consultant, where you’ll lead regulatory strategies and interact directly with regulatory agencies.
Is Regulatory Affairs Career Change Easy?
A job change can be hard – irrespective of the field you are working- so, a regulatory affairs career change too can be challenging, but at the same time rewarding too.
At the end of the day, it is all about your educational background and the way you crack the interview – which you can definitely land with the help of getting trained in the best Institute for PG Diploma in Clinical Research.
Why can regulatory affairs career progression be challenging?
1. Specialized knowledge: Regulatory affairs involve understanding regulations, laws, and guidelines in industries like pharmaceuticals, biotech, and medical devices. If you don’t have a background in these fields, it may take time to learn the necessary technical and regulatory knowledge.
2. Experience requirements: Many regulatory affairs roles expect candidates to have prior experience in a related field, such as clinical research, quality assurance, or legal compliance. Without this experience, it may be harder to enter directly into a Regulatory Affairs position.
3. Certifications and training: Employers may prefer candidates with certifications like RAC (Regulatory Affairs Certification). If you are thinking of regulatory affairs career progression from a different field, investing in such certifications may be necessary.
Buuut, that doesn’t mean that regulatory affairs career change or a regulatory affairs professional development is not possible or cannot be rewarding.

How regulatory affairs career change can be a rewarding experience?
- Transferable skills: Skills like attention to detail, communication, problem-solving, and project management are valuable in Regulatory Affairs. If you have experience in these areas, it can make the transition easier.
- Growing field: Regulatory Affairs is a growing field, especially in sectors like pharmaceuticals and medical devices. This can create more opportunities for newcomers willing to learn, making regulatory affairs career change easy!
- Public health and safety: Regulatory Affairs professionals ensure that products meet all safety and legal requirements before they reach the market. The role can be highly fulfilling as it directly impacts public health and safety, making regulatory affairs career progression a possibility.
How to ensure regulatory affairs professional development?
- Gain knowledge: Start learning about regulatory frameworks, such as FDA (US), EMA (Europe), or CDSCO (India), relevant to your target industry.
- Consider certifications: Look into Regulatory Affairs Certification (RAC) or other related courses.
- Get experience: If you have experience in related fields, highlight transferable skills in your applications.
- Network: Connecting with professionals in Regulatory Affairs through LinkedIn or attending industry events can open doors to job opportunities and mentoring.
On A Final Note…
If you are planning on becoming a regulatory affairs professional, it is important that you should possess a combination of education, industry experience, and specialized training. Enrolling in a clinical research course at a reputable clinical research institute provides an excellent foundation.
The investment in clinical research course fees will pay off as you gain the necessary qualifications and hands-on experience from the best institutes for PG Diploma in Clinical Research.
As global regulations evolve, professionals with expertise in clinical research and regulatory affairs will continue to play an important role in ensuring products meet safety standards and contribute to public health.