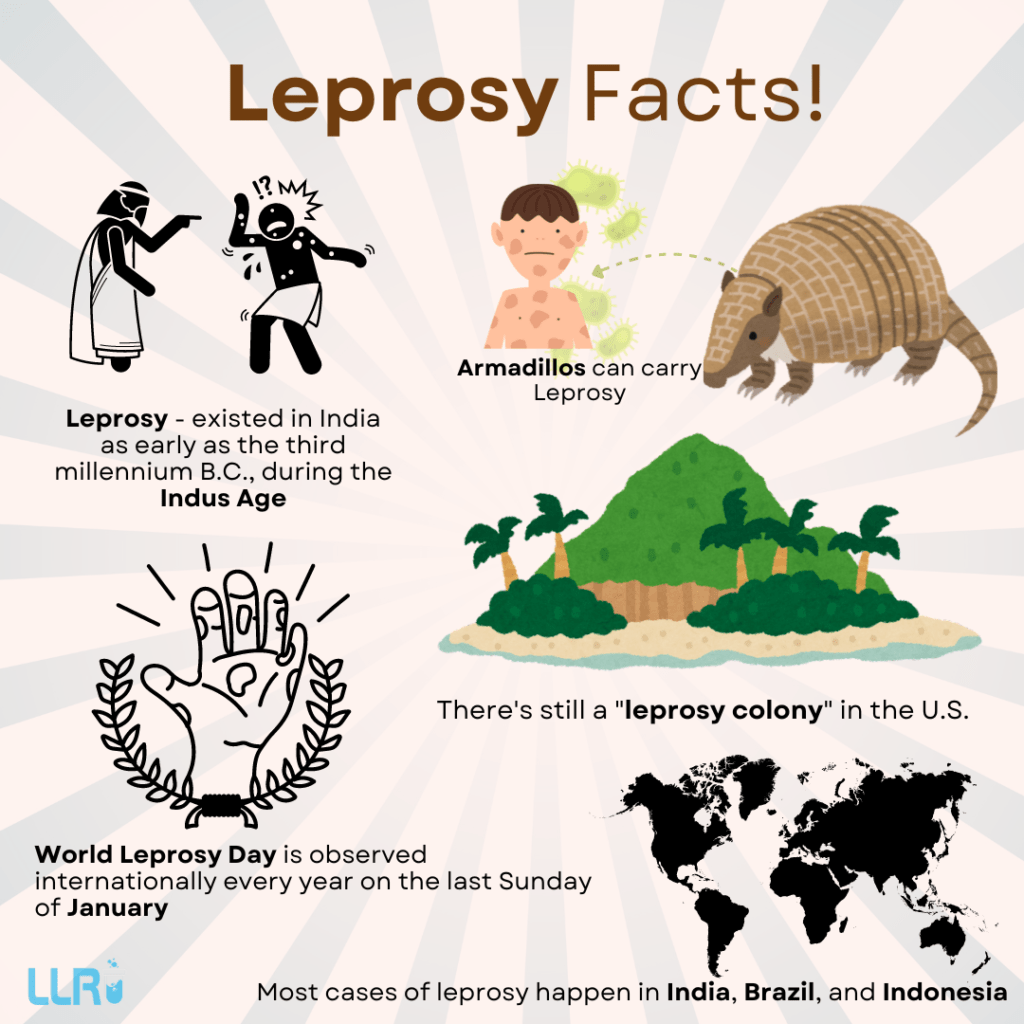
Leprosy Vaccine Research: Did you know that Leprosy existed in India as early as the third millennium B.C.? Yes, it was there during the Indus Age, roughly 5000 years ago from 2024. The ancient disease caused by the bacterium Mycobacterium leprae, has been a challenge for researchers and healthcare professionals for decades.
Leprosy, also known as Hansen’s disease, remains a significant public health issue in many parts of the world, including India. While progress has been made in controlling the disease, developing an effective vaccine remains a crucial step toward eradicating leprosy globally.
The current treatment for leprosy involves a combination of antibiotics known as multidrug therapy (MDT). This approach is effective in curing the disease and preventing transmission. MDT typically includes three key medications: rifampicin, clofazimine, and dapsone.
This blog will explore several aspects pertaining to Leprosy – from its vaccine, medicines to whether leprosy is curable and the current developments in leprosy treatment.
Here are the contents of the blog:
- Leprosy Vaccine Research
- The Role Of Patient Advocacy In Leprosy Research
- 5 Facts About Leprosy Vaccine Research
- New Developments In Leprosy Treatment
- Challenges In Leprosy Clinical Trials
- Leprosy FAQs
Leprosy Vaccine Research: What Is Going On Currently?
Current research into leprosy vaccines is making great improvement and paving way for change. Scientists are investigating new vaccine candidates beyond the traditional BCG vaccine, which has limited efficacy against leprosy.
Researchers are exploring recombinant vaccines (vaccines created using recombinant DNA technology) that use specific proteins from Mycobacterium leprae, the bacterium causing leprosy, to improve the immune response.
Clinical trials are underway to test the safety and effectiveness of these new vaccines, which is important for determining their potential in preventing leprosy in at-risk populations. Additionally, efforts are being made to improve the existing BCG vaccine.
Moreover, India is taking great steps when it comes to leprosy vaccine research. As per reports, after 36 years of testing, the experts in vaccine research introduced Indian-made leprosy vaccine. The indigenous vaccine is made from a heat-killed strain of bacteria known as Mycobacterium indicus pranii (MIP) or Mycobacterium w. This bacterium can be cultivated in a laboratory.
In the 1970s, Dr. G.P. Talwar and his team at the All India Institute of Medical Sciences in New Delhi developed this vaccine.
Let’s explore more into it below, in this blog.
The Role Of Patient Advocacy In Leprosy Research
Patient advocacy is integral to leprosy vaccine research. Experts highlight the importance of leprosy vaccine research, raising awareness about leprosy, support affected individuals, and make sure that the patients too have a say in the treatment and medicine research process.
- Recruitment: Advocates can help build trust within communities, encouraging individuals to participate in clinical trials.
- Improving access to care: By advocating for better healthcare access, patient advocates can help ensure that participants receive the care they need during trials.
- Raising funds: Advocacy groups often work to secure funding for leprosy research, addressing the financial constraints faced by researchers.
5 Facts About Leprosy Vaccine Research
With time, leprosy vaccine research is gaining momentum globally. The decline in leprosy cases around the world is proof of the improved treatment. Efforts are underway to accelerate leprosy vaccine research, particularly in endemic regions like India, Brazil, and Indonesia.
Here are 5 important facts about leprosy vaccine research:
1. Continuous research efforts: Leprosy vaccine research is a high priority due to the need for an effective vaccine to prevent the disease. Research efforts are focused on developing vaccines that can provide long-lasting protection and are suitable for use in endemic regions.
2. Development of multi-component vaccines: Researchers are exploring multi-component vaccines that target various aspects of the leprosy bacterium. These vaccines aim to stimulate a broader immune response and enhance protection against different strains of Mycobacterium leprae.
3. Collaboration with global health organizations: Leprosy vaccine research often involves collaboration between international health organizations, governments, and non-governmental organizations. This collaboration is crucial for pooling resources and expertise to accelerate vaccine development.
4. Advances in vaccine technology: Recent advancements in vaccine technology, including the use of recombinant DNA and adjuvants, are being applied to leprosy vaccine research. These innovations aim to improve vaccine efficacy and safety.
5. Clinical trials in endemic areas: To test new vaccines effectively, clinical trials must be conducted in areas with high leprosy prevalence. This requires careful planning and coordination to ensure the trials are conducted ethically and effectively.
New Developments in Leprosy Treatment
Beyond vaccine research, new developments in leprosy treatment are also being explored. The use of new drug combinations, the development of shorter treatment regimens, and improved diagnostics are part of this evolving landscape. The WHO’s strategy to eliminate leprosy includes not just treatment but also prevention through leprosy vaccine research, such as the following:
- Improved drug regimens: Research has led to the development of more effective drug regimens that reduce the duration of treatment and improve patient outcomes.
- Early detection techniques: Advances in diagnostic techniques are enabling earlier detection of leprosy, which is crucial for effective treatment and prevention of disease spread.
- Immunology developments: Recent advances in immunology have contributed towards understanding the immune response to Mycobacterium leprae better. This knowledge is being used to design more targeted vaccines and improve the overall approach to leprosy vaccine research.
- BCG vaccine: The BCG vaccine, originally used to prevent tuberculosis, has shown some effectiveness in preventing leprosy. Researchers are studying whether boosting with BCG could offer better protection and serve as a temporary solution while more specific leprosy vaccines are developed.
- Updated care approaches: New models of care that integrate medical, psychological, and social support are being offered to improve the overall well-being of individuals affected by leprosy.
What Are The Challenges In Leprosy Clinical Trials?
Conducting clinical trials for any disease is complex, but for leprosy, the challenges are complex and unique. Here are some key challenges in leprosy clinical trials:
- Stigma and misconceptions: Despite advances in treatment, leprosy still carries a social stigma. Many patients are hesitant to participate in trials due to fear of discrimination or rejection from their communities. The role of patient advocacy in leprosy research becomes important in encouraging participation and raising awareness.
- Long incubation period: Leprosy has an unusually long incubation period, sometimes lasting several years. This delays the onset of symptoms, making it difficult for researchers to detect early-stage disease and track vaccine efficacy over shorter time frames.
- Fewer disease prevalence: Thanks to successful treatment strategies, the prevalence of leprosy has decreased significantly. While this is positive, it also presents a challenge, as fewer cases make it harder to recruit participants for large-scale leprosy vaccine research.
- Biological complexity of the bacteria: Leprosy is caused by the slow-growing bacterium Mycobacterium leprae, which behaves differently from other bacteria. Its slow replication rate and complex nature pose difficulties in testing vaccines and treatments.
- Lack of animal models: Clinical trials often rely on animal models to simulate human disease and test vaccine responses. However, for leprosy, suitable animal models are limited, making it harder to predict how vaccines might work in humans.
On A Final Note…
While the challenges in leprosy clinical trials are significant, there is also optimism as leprosy vaccine research progresses. With new vaccines on the horizon and continued efforts from patient advocacy groups, the dream of eradicating leprosy is becoming more achievable. India, as one of the countries with a high burden of the disease, plays a critical role in supporting this research and advancing new developments in leprosy treatment.
Moreover, with increased awareness, participation, and innovation, the future of leprosy vaccine research holds great promise for a leprosy-free world.