Protocols for clinical trials
The genesis of every clinical inquiry involves crafting a clinical trial protocol—a document delineating the trial’s objectives, design, methodology, statistical nuances, and organizational structure. Its paramount purpose is to safeguard trial subjects and uphold the integrity of the collected data, providing a succinct roadmap for the trial’s execution.
Clinical research protocols
A clinical research protocol is a comprehensive document outlining the background, rationale, objectives, design, enrollment criteria, methodology, data recording requirements, statistical considerations, and study organization for a clinical research investigation.
The protocols for clinical trials precisely outlines the study’s conduct, establishing consistent standards for researchers. This uniformity enables the aggregation of data from various sites, ensuring the attainment of valid conclusions.
Protocol review evaluates whether a study can be feasibly completed with available resources and capabilities.
Clinical practice guidelines
Clinical practice guidelines, commonly known as “clinical guidelines,” represent expert recommendations delineating the most effective approaches for the diagnosis and treatment of medical conditions. Although principally tailored for physicians, these guidelines extend their relevance to nurses and various healthcare professionals.
Clinical guidelines are vital for ensuring patients receive appropriate and holistic treatment, such as those for cervical cancer. Beyond diagnosis and treatment, they encompass crucial aspects like psychosocial support, rehabilitation, and follow-up care.
Guidelines synthesize medical knowledge, weighing the merits of diagnostics and treatments. They provide clear recommendations based on scientific evidence, requiring regular updates for ongoing relevance.
Unlike directives, guidelines lack legal obligations. Physicians have the autonomy to deviate from recommendations if deemed unfit for specific patients, but any deviations should be justified with valid reasons.
What is the process involved in the development of clinical practice guidelines?
Ideally, the development of clinical guidelines should adhere to a systematic approach, meaning it follows a specific procedure:
- The initial step involves establishing a guideline committee, which is typically led by a representative from the relevant medical association. This committee comprises specialists who collectively cover the essential aspects of the medical condition in question.
- The committee gathers extensive information from diverse sources and evaluates it using predetermined criteria. During the guideline-writing process, the committee considers various assessments and opinions of its members, creating recommendations that are essentially “consensus-based.”
- Committee members are required to disclose any conflicts of interest, such as whether they have been affiliated with a pharmaceutical company producing medication for the specific medical condition addressed by the clinical guidelines in development.
Clinical trial protocols examples
Clinical trials protocols, sponsored by various entities, serve as a method to study a broad spectrum of interventions, including new or existing medicines, medical devices, and other medical or non-medical approaches.
For example, a clinical trial could involve new drugs, medical devices, biologicals, vaccines, surgical and other medical treatments and procedures. Psycho-therapeutic and behavioural therapies help service changes, preventative care strategies and educational interventions are also examples of clinical trials-reframe. Researchers might also conduct clinical trials to evaluate diagnostic or screening tests and new ways to detect and treat disease.
4 phases of clinical trial
Clinical trials progress through phases, with early stages emphasizing safety and side effects of a new drug. In later phases, the focus shifts to evaluating the comparative effectiveness against existing standards.
Clinical trials are typically categorized into three main phases: Phase 1, Phase 2, and Phase 3. Phase 1 trials represent the earliest stage, while Phase 3 trials are considered later-stage evaluations.
Certain trials include an initial stage known as Phase 0, and beyond the standard phases, there are Phase 4 trials conducted after a drug has been licensed.
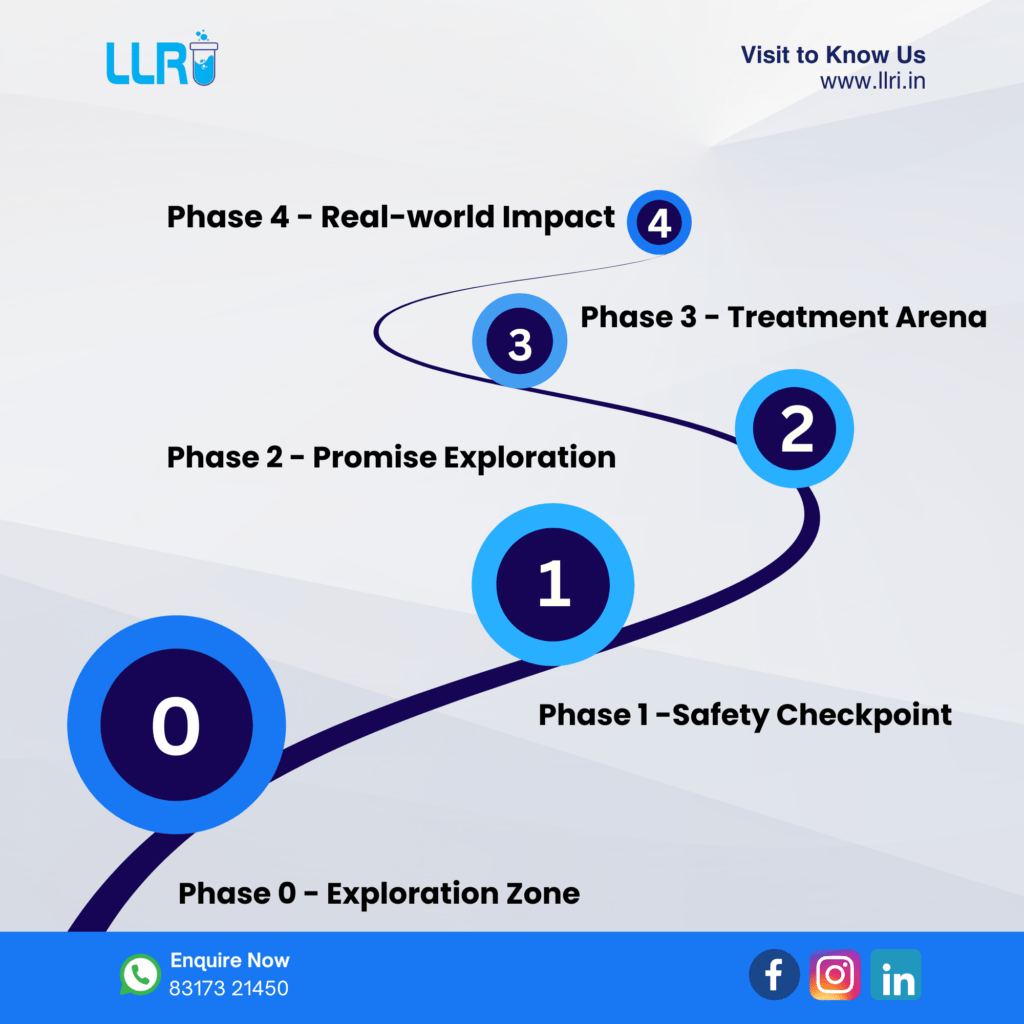
Phase 0 trials
Primarily, early human trials are Phase 1 studies, yet your doctor may suggest Phase 0 participation to validate a drug’s behavior from lab research. In these studies, a small group receives a minimal drug dose, not for treatment but to minimize potential side effects.
Phase 0 trials aim to find out things such as:
- Determining if the drug reaches cancer cells.
- Tracking the drug’s journey in the body.
- how cancer cells in the body respond to the drug.
Phase 1 trials
Phase I trials, often denoted as Phase 1, are typically small-scale studies that enroll only a handful of participants. These trials may extend invitations to individuals facing various advanced cancers, particularly those who have exhausted all existing treatment options.
Phase 1 trials seek to determine essential information:
- Determine the safe dosage of the drug.
- Identify the potential side effects.
- Examine the drug’s fate within the body.
- Assess if the treatment effectively reduces cancer size.
Phase 1 trials advance slowly with careful patient selection, emphasizing dose escalation. Starting with minimal drug doses, researchers gradually increase them while monitoring side effects and participant well-being. Frequent blood tests assess drug handling and elimination. The primary goal is to discern optimal doses and side effects, laying the foundation for efficacy assessments. While some may benefit, the primary objective is advancing scientific understanding.
Phase 2 trials
Phase 2, or Phase II, trials follow Phase 1, but not all Phase 1 treatments advance to this stage. Phase 2 trials may focus on a specific cancer type or include individuals with various cancers.
Phase 2 trials seek to determine essential information:
- If the new treatment shows promise, it advances to a larger Phase 3 trial for further testing.
- Identify the specific cancers the treatment effectively targets.
- Explore side effects and their management strategies.
- Determine the optimal dosage for administration.
Following Phase 1, unforeseen side effects may arise in treatment due to individual variations. Though some may benefit, results aren’t uniform. Phase 2, with approximately 100 participants, entails comparisons with existing treatments or placebos, and randomization is employed in some trials for unbiased participant allocation.
Phase 3 trials
Phase 3, or Phase III, trials compare new treatments directly with the current best standard treatment.
Phase 3 trials seek to determine essential information:
- Identify the superior treatment for a specific cancer type.
- more about the side effects
- how the treatment affects people’s quality of life
They may compare standard treatment with:
- a completely new treatment
- different doses of the same treatment
- having the same treatment more, or less, often
- An innovative approach to administering a standard treatment, like radiotherapy, is being explored.
Phase 3 trials, involving significantly more patients than Phases 1 or 2, require larger numbers to detect small success rate differences. These trials often include thousands across various hospitals and countries, mostly being randomized for unbiased treatment group allocation.
Phase 4 trials
Phase 4, or Phase IV, trials occur after a drug has proven efficacy and received licensing.
Phase 4 trials seek to determine essential information:
- Examine the drug’s side effects, including rare occurrences, and evaluate its overall safety profile.
- what the long term risks and benefits are
- how well the drug works when it’s used more widely for people not included in the phase 3 trial
Conclusion
Clinical investigations thrive on meticulous trial protocols, vital for safety and data integrity. This document, outlining objectives and methodology, ensures seamless execution, contributing to the inquiry’s success. Clinical practice guidelines extend expert recommendations to diverse healthcare professionals. Sponsored clinical trial protocols study a wide array of interventions, progressing from safety emphasis to comparative effectiveness evaluation in Phases 1 to 3. Certain trials include Phase 0 and Phase 4, enriching the spectrum of research stages.

