Regulatory Affairs for Pharma: The pharmaceutical industry is one of the most regulated sectors in the world – so it doesn’t take a genius to know the important role regulator affairs play in there! Regulatory affairs professionals act as the bridge between the pharmaceutical companies and the regulatory authorities.
Did you know that for pharma students, this field offers exciting career opportunities that combine science, law, and communication?
Regulatory Affairs for Pharma: What Is Regulatory Affairs in Pharma?
Regulatory affairs for pharma involve overseeing the approval of new pharmaceutical products, ensuring compliance with safety standards, and managing documentation for drug approvals.
These professionals are responsible for navigating the complex regulatory landscape, which includes agencies like the US FDA, EMA in Europe, and CDSCO in India.
In simpler terms, regulatory affairs professionals make sure that pharmaceutical products, from medicines to vaccines, are safe and effective for consumers. They are involved in every stage, from clinical trials to marketing approval.
Pharma students often ask, “Why should I pursue a career in regulatory affairs for pharma students?” The answer lies in the significant impact these roles have on public health and the global pharmaceutical market.
Why Should Pharma Students Consider Regulatory Affairs?
For pharma students, regulatory affairs are an ideal career path because it offers diverse roles and responsibilities. It’s a field that requires not just scientific knowledge, but also excellent communication and project management skills.
Regulatory affairs for pharma students are in demand, especially with the rise of new and complex medications.
According to recent studies, the global pharmaceutical market is projected to grow by over 5% annually, creating new job opportunities in regulatory affairs jobs for pharmacists and other pharma professionals.
Top Regulatory Affairs Jobs in the Pharmaceutical Industry
The regulatory affairs jobs pharmaceutical industry offers several career paths for those who are interested. Here are some top positions that pharma students can consider in the field of regulatory affairs:
1. Regulatory Affairs Specialist: A Regulatory Affairs Specialist ensures that all pharmaceutical products comply with national and international regulations. This role involves preparing and submitting regulatory documents to authorities.
2. Regulatory Affairs Manager: With experience, you can move into a managerial position, where you’ll oversee the regulatory submissions process, coordinate with regulatory bodies, and manage a team of specialists.
3. Clinical Regulatory Affairs Associate: This role focuses on clinical trials, making sure they meet regulatory standards. Clinical regulatory affairs professionals ensure that all trial data is collected and submitted correctly to regulatory bodies.
4. Quality Assurance Regulatory Affairs Officer: In this role, you work closely with the quality assurance team to ensure that all pharmaceutical products meet the highest safety and quality standards.
5. Drug Regulatory Affairs Specialist: As a specialist in drug regulatory affairs, your job will involve filing drug approval applications, handling post-approval changes, and ensuring that all regulatory submissions are accurate.
What Are The Skills Required for Regulatory Affairs Jobs?
To excel in regulatory affairs jobs for pharmacist, you’ll need a mix of technical and soft skills. Here are some of the key skills you should develop:
- Attention to detail: Regulatory work requires a sharp eye for detail, as you’ll be responsible for ensuring compliance with numerous guidelines.
- Project management: Managing submissions and coordinating with various departments requires strong organizational and time-management skills.
- Communication skills: Since regulatory professionals often liaise with government bodies, clear and concise communication is critical.
- Scientific knowledge: A strong background in pharmacology or life sciences is essential for understanding the products you’re working with.
Career Growth in Regulatory Affairs
The field of regulatory affairs for pharma offers immense career growth. As companies expand and develop more complex drugs, the demand for regulatory professionals continues to increase. Furthermore, regulations are constantly changing, making it a dynamic and challenging career option for pharma students.
“Regulatory affairs is one of the most rewarding career paths in the pharmaceutical industry,” says Dr. Pooja, an expert in regulatory sciences. “Not only do you get to work on life-saving drugs, but you also play a crucial role in bringing them to market.”
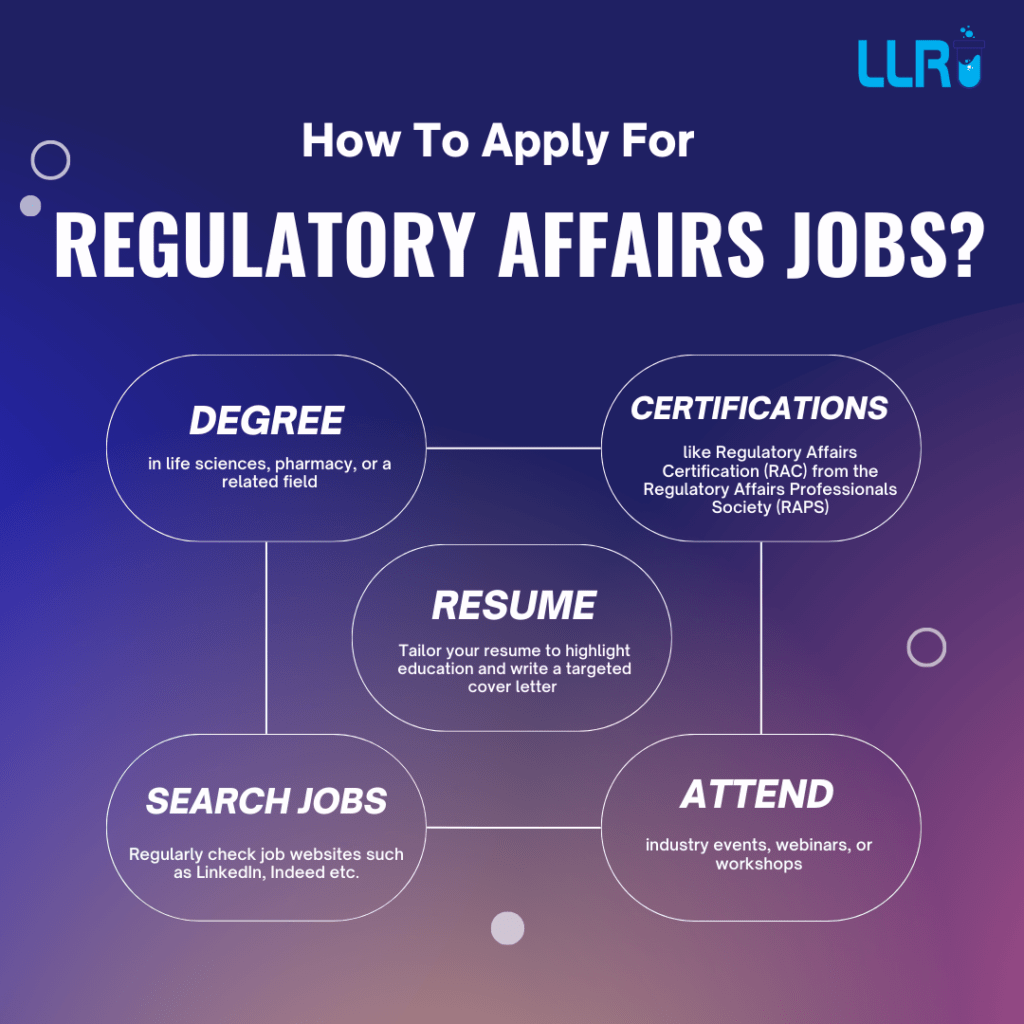
How To Apply For Regulatory Affairs Jobs?
To apply for Regulatory Affairs (RA) jobs in the pharmaceutical, biotechnology, or healthcare sectors, follow these steps, generally:
- Step 1: Obtain a degree in life sciences, pharmacy, or a related field. If possible, pursue a diploma or advanced degree in regulatory affairs to boost your qualifications.
- Step 2: Internships or entry-level roles in pharmaceutical companies, clinical trials, or quality assurance will provide useful exposure to the field.
- Step 3: Consider getting a Regulatory Affairs Certification (RAC) from the Regulatory Affairs Professionals Society (RAPS) to stand out.
- Step 4: Tailor your resume to highlight education, skills, and any experience related to regulatory affairs (e.g., knowledge of FDA/EMA regulations, quality assurance, clinical trials).
- Step 5: Write a targeted cover letter emphasizing your interest in regulatory compliance, your understanding of the regulatory landscape, and how your skills meet the job requirements.
- Step 6: Search for jobs in online job portals like LinkedIn, Indeed, Naukri.com (for Indian market), Glassdoor, PharmiWeb, BioSpace (for biotech/pharma jobs) etc.
- Step 7: Many pharmaceutical companies, biotech firms, and healthcare organizations list job openings on their official career pages. Some key companies include Pfizer, Novartis, GSK, Sanofi, Roche.
- Step 8: LLRI also has a job portal which you could access once you’ve joined our courses, which would provide you with direct access to job openings, as we are internal hiring partners to several MNCs.
- Step 9: If you’re just starting out, look for Regulatory Affairs internships or junior roles. Many companies offer entry-level regulatory positions that allow you to grow into more senior roles over time.
- Step 10: Prepare for interviews, be well-versed in regulatory guidelines (FDA, EMA, MHRA, etc.). Understand the pharmaceutical or medical device product development process and practice explaining how your experience or education relates to compliance, submissions, and dealing with regulatory bodies.
- Step 11: Follow the application instructions on job postings carefully. Apply to multiple positions to increase your chances, but tailor each application to the specific role.
What Are The Future Prospects for Regulatory Affairs in Pharma?
As the pharmaceutical industry continues to grow, so does the need for professionals in regulatory affairs for pharma. With the global push for faster drug development and approval, regulatory professionals are becoming an integral part of the process.
“The demand for regulatory professionals is higher than ever, especially in emerging markets like India,” says reports. With companies expanding their operations globally, understanding international regulatory standards is very important. LLRI’s programs are designed to equip you with the skills and knowledge you need to succeed in regulatory affairs jobs pharmaceutical industry.
One of our recent graduates, Acshah Sujith, who completed the course, said, “The LLRI program gave me the knowledge and confidence to land my first in a reputed pharma company.”
And with LLRI courses that are available both online and offline, you get these benefits, which can definitely help you land a job quicker than your counterparts:
- Live projects that give you practical exposure to regulatory processes.
- Mentorship from industry experts with decades of experience.
- Job placement assistance in top pharmaceutical companies.

On A Final Note…
For pharma students looking to build a rewarding and stable career, regulatory affairs for pharma are a field that offers plenty of opportunities. Whether you’re interested in ensuring the safety of clinical trials, managing drug approvals, or navigating complex international regulations, there’s a role for you.
With the right training and qualifications, you can find a fulfilling career in regulatory affairs jobs for pharmacist roles, helping to bring life-saving drugs to market and ensuring that they meet the highest safety standards.

