SDTM Interview Questions: For freshers stepping into the world of clinical research, SDTM is as important as it gets. Study Data Tabulation Model is central to clinical trial data, and it works in conjunction with Clinical SAS to standardise and organise data. SDTM interview questions are commonly asked to assess a candidate’s knowledge of this critical process, which helps assure compliance with regulatory requirements.
What Is The Link Between Clinical SAS and SDTM?
Before we get into the SDTM interview questions, let’s understand connection between Clinical SAS and SDTM. In clinical research, SAS (Statistical Analysis System) is a powerful tool used for managing and analysing data. When it comes to submitting clinical trial data to regulatory bodies like the FDA, the data needs to follow specific standards.
That’s where SDTM comes into play.
SDTM is a standardised format developed by the Clinical Data Interchange Standards Consortium (CDISC) to ensure that clinical trial data is organised and consistent. Clinical SAS is used to program and format data according to SDTM guidelines. In simple terms, SDTM provides the structure, and Clinical SAS helps implement that structure by transforming raw clinical data into a standardised form.
The connection between Clinical SAS and SDTM is important because SDTM helps organise data in a clear, structured way. By following SDTM standards, researchers can make sure their data is consistent and easy to work with in Clinical SAS. This makes it simpler to manage, analyse, and understand the data, while also speeding up the process of submitting it to regulatory bodies. Overall, it improves the quality and consistency of clinical research data.
Hence, having a good grasp of SDTM and Clinical SAS is essential for freshers looking to enter clinical research roles.
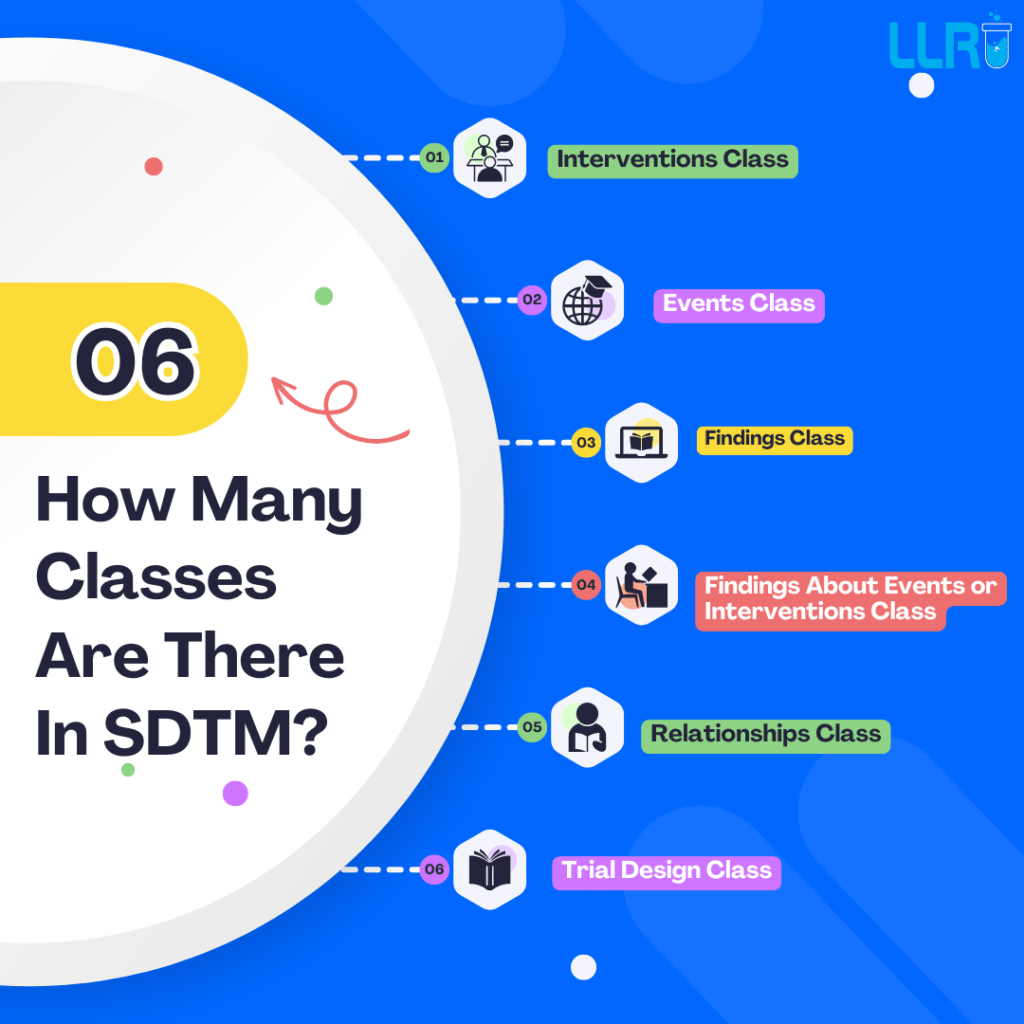
Common SDTM Interview Questions For Freshers
If you are a fresher preparing for an SDTM interview, you must be ready to answer questions related to SDTM concepts, its connection to Clinical SAS, and how the model works in practice.
Below are some frequently asked SDTM interview questions and answers for freshers.
1. What is SDTM, and why is it important?
Answer: SDTM, or Study Data Tabulation Model, is a standard created by CDISC to ensure that clinical trial data is submitted in a structured and consistent format. It is important because regulatory bodies like the FDA require clinical trial data to be in SDTM format for submission and approval processes. SDTM ensures the integrity, consistency, and transparency of clinical data.
2. How does Clinical SAS work with SDTM?
Answer: Clinical SAS is used to process and transform raw clinical trial data into SDTM-compliant datasets. SAS programmers create the datasets according to SDTM guidelines, ensuring the data is in the proper format for submission. The combination of Clinical SAS and SDTM ensures that data is properly organised for regulatory review.
3. What are the key components of SDTM?
Answer: The key components of SDTM include:
- Domains: Specific datasets that represent different types of data, like adverse events (AE), medical history (MH), or lab data (LB).
- Variables: The columns within a dataset, such as subject ID, visit date, and test results.
- Controlled Terminology: Standard terms used to ensure consistency in the data across studies and submissions.
4. Why is it essential for freshers to understand SDTM domains?
Answer: Understanding SDTM domains is critical because they define how different types of clinical data are organised. Each domain represents a specific dataset, and knowing how to correctly classify data into these domains is a key skill for anyone working in clinical research. SDTM interview questions for freshers often focus on domain knowledge, so it’s important to be well-versed in the common SDTM domains.
5. What challenges can you face while creating SDTM datasets?
Answer: Challenges include:
- Mapping raw data to SDTM standards: Ensuring that the data collected in the clinical trial is properly mapped to SDTM domains.
- Understanding controlled terminology: Applying the correct standard terms can be complex for beginners.
- Handling data discrepancies: Sometimes, raw data may have inconsistencies that need to be resolved before it can be transformed into SDTM format.
6. What is the difference between SDTM and ADaM?
Answer: SDTM is used for tabulating clinical trial data, ensuring that it is consistent and structured according to CDISC standards. ADaM (Analysis Data Model), on the other hand, is used for datasets that are created specifically for statistical analysis. While SDTM is more focused on standardisation, ADaM allows for greater flexibility for analysis purposes.
7. How do you ensure the quality of SDTM datasets?
Answer: Quality is ensured through rigorous validation processes, including:
- Double-checking data mapping to SDTM domains.
- Running SDTM compliance checks using tools like Pinnacle 21.
- Collaborating with clinical teams to resolve any data discrepancies.
8. How are SDTM datasets structured?
Answer: SDTM datasets are structured in a two-dimensional format with rows representing individual subjects or events and columns representing variables. This tabulation ensures consistency and standardization across studies.
9. What is the role of the AE domain in SDTM?
Answer: The AE (Adverse Events) domain records any adverse events that occur during the clinical trial. It is crucial for tracking the safety and side effects of a drug.
10. What are some common issues you may face when implementing SDTM standards?
Answer: Common issues include handling missing data, ensuring data quality, and mapping non-standard variables into the SDTM-compliant format.

11. What tools are commonly used for SDTM mapping?
Answer: SAS, Pinnacle 21, and other data transformation tools are commonly used for SDTM mapping and validation.
12. How does SDTM benefit regulatory authorities?
Answer: SDTM simplifies the review process for regulatory authorities like the FDA by providing a consistent, standard format, ensuring faster and more accurate data analysis.
13. What is a domain in SDTM?
Answer: A domain in SDTM is a collection of data related to a specific aspect of a clinical study, such as Demographics (DM), Adverse Events (AE), and Vital Signs (VS).
14. What is the role of the LB domain in SDTM?
Answer: The LB (Laboratory Tests) domain contains data from laboratory tests conducted during the clinical trial. It includes results like blood counts, liver function tests, and other diagnostic measures.
15. What is the significance of the VS domain in SDTM?
Answer: The VS (Vital Signs) domain captures data related to a patient’s vital signs, such as heart rate, blood pressure, and temperature, crucial for monitoring patient health during the trial.
16. What are the steps involved in converting raw data to SDTM format?
Answer: The steps include:
- Data extraction from clinical trial databases.
- Mapping the data to SDTM domains.
- Validating the data to ensure it meets SDTM standards.
- Submitting the data to regulatory authorities.
17. What is the significance of the RELREC domain?
Answer: The RELREC (Related Records) domain is used to define relationships between records in different datasets, ensuring accurate representation of the data relationships.
18. What are supplemental qualifiers in SDTM?
Answer: Supplemental qualifiers are used to store additional information not covered by standard SDTM domains. They are placed in the SUPPQUAL datasets and referenced back to the main domain.
19. Why is SDTM validation necessary?
Answer: Validation ensures that SDTM datasets meet regulatory requirements and are free of errors. Tools like Pinnacle 21 are commonly used to validate SDTM datasets.
20. What is the role of SDTM in clinical data submissions?
Answer: SDTM plays a crucial role in ensuring clinical trial data is submitted in a standardized format, which is essential for regulatory approvals from bodies like the FDA and EMA.

On A Final Note…
Preparing for an interview involving SDTM can be daunting, but with a solid understanding of SDTM interview questions and their answers, you can confidently showcase your knowledge.
SDTM, combined with Clinical SAS, forms the backbone of clinical data management, and expertise in these areas will set you on the right path for a successful career in clinical research.
After all, a thorough knowledge of SDTM standards and their practical applications will set you apart in the field of clinical research for sure!

