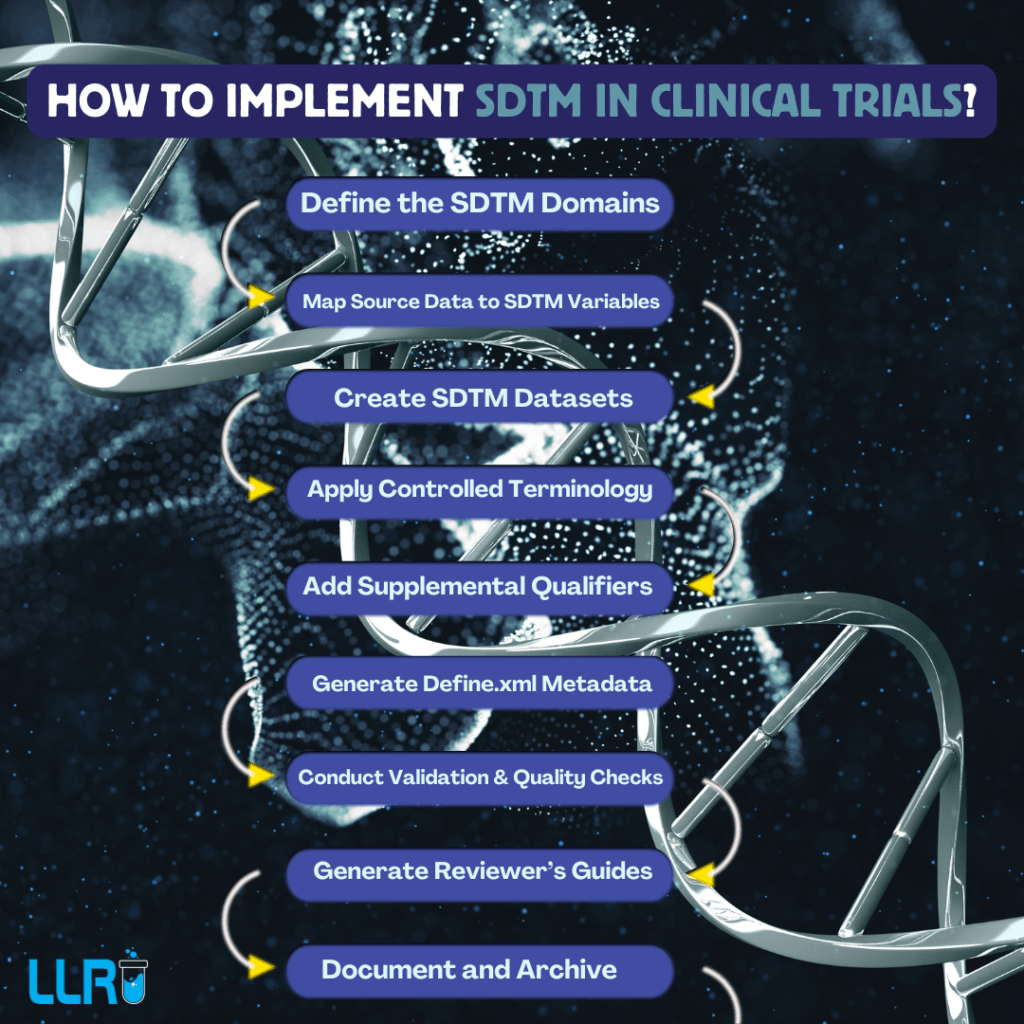
All About SDTM Programming: The influence SDTM programming has in the realm of clinical trial data management cannot be ignored. By organising the data in an accurate manner and in compliance with regulatory standards, SDTM programming software really is a life saver for many!
What Is SDTM Programming Software?
SDTM programming software is designed to help researchers and data managers in converting clinical trial data into a format that is in compliance with the SDTM guidelines that are set by the regulatory authorities, such as the FDA and EMEA.
This software plays an important role in transforming raw clinical trial data into standardized formats that can be easily reviewed and analyzed.
What Are The 7 Key Features To Look For In SDTM Software?
When selecting SDTM programming software, several key features should be considered to ensure it meets your needs effectively:
1. Compliance with Regulatory Standards
One of the most critical aspects of SDTM programming software is its ability to comply with regulatory standards. The software should be capable of producing data in the SDTM format that adheres to the latest guidelines from regulatory bodies. This feature ensures that your submissions are in line with industry standards, reducing the risk of rejections or delays.
2. User-friendly Interface
A user-friendly interface is crucial for efficient use of SDTM programming software. The software should be intuitive and easy to navigate, allowing users to perform tasks without extensive training. Features like drag-and-drop functionality, customizable views, and clear navigation paths enhance the user experience.
3. Integration Capabilities
The software should offer robust integration capabilities with other data management systems and tools. This feature enables seamless data transfer between different platforms, reducing manual data entry and potential errors. Integration with popular statistical analysis tools and electronic data capture systems can streamline workflows significantly.
4. Support for Multiple Data Formats
Look for SDTM programming software that supports a variety of data formats. This flexibility allows users to handle data from different sources and convert it into the required SDTM format with ease. Support for formats such as SAS, CSV, and Excel can be particularly beneficial.
5. Customisation Options
Customisation options are important for tailoring the software to meet specific project needs. The ability to create custom mappings, define unique variables, and adjust settings according to project requirements can greatly enhance the efficiency of data processing.
6. Technical Support and Documentation
Reliable technical support and comprehensive documentation are essential features. Good SDTM programming software should offer access to support resources, including help desks, user manuals, and online tutorials. This support is crucial for resolving issues quickly and ensuring smooth operation.
7. Open-Source Options
For those interested in cost-effective solutions, open-source SDTM programming software can be an excellent choice. These options often provide flexibility and can be customised to suit specific needs without the cost associated with commercial software.
5 Most Popular SDTM Programming Software
Here’s a table highlighting some of the most popular SDTM programming software solutions, both commercial and open-source:
| Software Name | Type | Key Features |
| SAS Clinical Data Integration | Commercial | CDISC-compliant, automation, robust validation tools, integrates with SAS. |
| Pinnacle 21 | Open-source | Industry-standard validation tools, easy-to-use interface, comprehensive reports. |
| Medidata Rave | Commercial | Full integration with clinical trial systems, automation, CDISC compliance. |
| OpenCDISC Validator | Open-source | Free, extensive validation features, user-friendly, widely used in the industry. |
| PhUSE SDTM Toolkit | Open-source | Community-driven, supports SDTM conversions, customizable templates. |
Open-Source SDTM Software: A Growing Trend In Clinical Research
As clinical research continues to evolve, the use of open-source SDTM software is becoming more prevalent. Open-source options like Pinnacle 21 and OpenCDISC Validator offer powerful, community-driven tools that are cost-effective and frequently updated to meet industry standards.
These tools are great for small to mid-sized organizations that may not have the budget for high-end commercial software but still require robust SDTM capabilities. So, what are these really?
What are open-source SDTM software?
Open-source SDTM (Study Data Tabulation Model) software is a fantastic alternative to expensive commercial tools for managing clinical trial data. It provides the necessary features to secure compliance with SDTM standards, while offering flexibility and customisation options without the high costs associated with proprietary software.
Open-source SDTM software is typically community-driven, meaning it benefits from ongoing contributions from developers and users worldwide. This results in continuous improvements and updates to meet the evolving needs of clinical data management.
Here are some common open-source SDTM software options:
OpenCDISC: OpenCDISC is one of the most well-known open-source tools for SDTM compliance. It offers features for SDTM validation, making it easier to check for errors and ensure that your data complies with regulatory standards. Its flexibility allows users to adapt the tool to specific project needs, and it integrates well with other systems, making it a go-to choice for many.
R (with Custom Libraries): R is a popular open-source statistical software that can be used for SDTM programming with the help of custom libraries. R offers tremendous flexibility and is widely used in clinical trials for data analysis, reporting, and validation. By using relevant libraries, R can convert clinical trial data into SDTM formats while providing powerful statistical analysis capabilities.
CDISC Validator: CDISC Validator is another open-source SDTM software that checks data for compliance with CDISC standards, including SDTM. It allows users to validate datasets against specific rules and regulatory guidelines, helping to reduce submission errors.

On A Final Note…
When managing and analysing the clinical trial data, one of the primary factors is picking the right SDTM programming software. By focusing on key features like CDISC compliance, automation, validation, and integration capabilities, organizations can streamline their data processes and ensure regulatory success.
Moreover, always consider what are the key features to look for in SDTM software to make sure that your clinical data submission is smooth, fast, and accurate – after all, who wants the data collection and analysis and everything that comes with it to be messy, right?!

